[[4.2 Features and Climates of an ecosystem]]
*Rainforest
- high hummidity
- 29-30
- range = 4 degrees
- rain all year
- 2000 mm rain per year
- Tall, broad evergreen trees
- canopied forests with layered structure
- rapid nutrient cycling
- biomas 45.0jg/m
*Savanna Forest
- 23-33
- range = 10 degrees
- 10 months of rainfall
-800-900mm rain per year
- mixed grasslands with trees
- clearings of woody shrubs and tall trees
- drought resistant trees
- biomass 9.0kg/m
*Savanna Grassland
- 23-33
- range = 10 degrees
- 7 months of rainfall
-800-900mm rain per year
- mixed grasslands with trees
- clearings of woody shrubs and tall trees
- drought resistant trees
- biomass 9.0kg/m
*Sahel Scrubland/Semi Desert
- 22-36
-range=14 degrees
- 3-5 months of rainfall
- 300-500 mm rain per year
- Tall Savanna grass
-Isolated drought
- protection agaisnt fire/animal predators
- biomas 4.0
*True Desert
- 10-40
- range = 30 degrees
- 0mm annual precipitaion
- low humidity
- 0-100 (flash floods) rain per year
- Xerophytic plants to adapted to arid conditions
- limited species
- Biomas 0.6 kg/m
Friday, 8 June 2007
Geography [[Weather and Climate]] 4.2
[[4.2 What management problems does the seasonality of climates cause?]]
Life styles are completely controlled by the need to find water, and the need to know where the nearest supply of water is should the original source fail.
Thus, one of the main management problems is the management of water.
There are three types of Rivers:
1)Ephermeral Stream: desert/semi desert. Irregular flow in short episodes
2) Intermittent Streams: wet/dry seasons with relaible patterns
3) Perennial Streams: sustains base flow with source in wet area.
Coping with different rainfall patterns: different strategies
1) Storage
- store of water or food - underground river tapping
2) Migration/Evacuation
- Leave areas fir period when rain stops
3) Technology strategy
- transport water from ekse where
Management Case Studies:
Equitorial Climate:
*Hunters and Gatherers
* Slash and Burn
Savanna
*The Maasai
- water obtained from springs fed by snowmelt from Kilimajaro
- water sources are seasonal
- major droughts occur as deficit is present in water budget
- tribes move seasonally
Semi Desert
*Burkina Faso
- threat of droughts or famine
- rainfall confined to 2 months
- length and total rainfall unreliable
*North Kenya
- rainfall too low and unreliable to support settled agriculture
- Rain in heavy localised downpours
- Tribes migrate to new grass growth after downpour
Mediterranean
*The Nile
- Annual Flooding
- Summers dry but too hot
- Labour intensive
- Aswan Damn built to allow control of water
- Technology strategy/Storage
- no annual deposition of fertile silt by flood water so fertislisers needed.
Life styles are completely controlled by the need to find water, and the need to know where the nearest supply of water is should the original source fail.
Thus, one of the main management problems is the management of water.
There are three types of Rivers:
1)Ephermeral Stream: desert/semi desert. Irregular flow in short episodes
2) Intermittent Streams: wet/dry seasons with relaible patterns
3) Perennial Streams: sustains base flow with source in wet area.
Coping with different rainfall patterns: different strategies
1) Storage
- store of water or food - underground river tapping
2) Migration/Evacuation
- Leave areas fir period when rain stops
3) Technology strategy
- transport water from ekse where
Management Case Studies:
Equitorial Climate:
*Hunters and Gatherers
* Slash and Burn
Savanna
*The Maasai
- water obtained from springs fed by snowmelt from Kilimajaro
- water sources are seasonal
- major droughts occur as deficit is present in water budget
- tribes move seasonally
Semi Desert
*Burkina Faso
- threat of droughts or famine
- rainfall confined to 2 months
- length and total rainfall unreliable
*North Kenya
- rainfall too low and unreliable to support settled agriculture
- Rain in heavy localised downpours
- Tribes migrate to new grass growth after downpour
Mediterranean
*The Nile
- Annual Flooding
- Summers dry but too hot
- Labour intensive
- Aswan Damn built to allow control of water
- Technology strategy/Storage
- no annual deposition of fertile silt by flood water so fertislisers needed.
Geography [[Weather and Climate]] 4.2
[[4.2: The relationship between Climate and Seasonality]]
Ecosystems: natural units in which the life styles and cycles of plants and animals are linkedto each other and to the non-living constituents of the environment to form natural living systems.
Natural Vegetation (Climax Community): Vegetation which will develop which is dominiated by plants which, of all those avaliable can
compete most successfully in the exsisting physical environment.
Biomes: Large global ecosystems relted to major climate zones.
The world Biomes:
* Tundra *Temperate Grassland *Tropical Rainforest *Taiga *Evergreen woodland *Savanna *Temperate deciduous forest *Desert
Adaptions of vegetation to Climate
- In sufficient rainfall, trees dominate
- the higher the rainfall and temperature the taller the tees and the larger the leaves
- higher the rainfall and the temperature the higher the rate at which plants produce organic matter
- in low or seasonal rainfall, plants develop xerophytic features
*Reduced leaf areas
*Sunken Stomata
*Long root system
*Slow growth rate
*Fire resistant trunks and seds
*Root storage system
- In seasonal climates, plants loose their leaves.

Ecosystems: natural units in which the life styles and cycles of plants and animals are linkedto each other and to the non-living constituents of the environment to form natural living systems.
Natural Vegetation (Climax Community): Vegetation which will develop which is dominiated by plants which, of all those avaliable can
compete most successfully in the exsisting physical environment.
Biomes: Large global ecosystems relted to major climate zones.
The world Biomes:
* Tundra *Temperate Grassland *Tropical Rainforest *Taiga *Evergreen woodland *Savanna *Temperate deciduous forest *Desert
Adaptions of vegetation to Climate
- In sufficient rainfall, trees dominate
- the higher the rainfall and temperature the taller the tees and the larger the leaves
- higher the rainfall and the temperature the higher the rate at which plants produce organic matter
- in low or seasonal rainfall, plants develop xerophytic features
*Reduced leaf areas
*Sunken Stomata
*Long root system
*Slow growth rate
*Fire resistant trunks and seds
*Root storage system
- In seasonal climates, plants loose their leaves.

Geography [[Weather and Climate]] 4.2
[[4.2 Why seasonal climates and how does the effect vegetation?]]
The Climates:
Mediterranian
Hot Desert
Savanna
Equitorial Climate
Mediterranian:
July= subtropical high pressure leading to hot and dry climate
December = Polar Low Pressure leaidng to warm and wet climate
Hot Desert
July = Subtropical high pressure leading to hot and dry climate
December = Subtropical high pressure leading to hot and dry climate
Savanna
July = Equitorial LowPressure leading to hot and wet climate
December = Subtropical high pressure leading to hot and dry climate
Equitorial Climate
July = Equitorial LowPressure leading to hot and wet climate
December = Equitorial LowPressure leading to hot and wet climate
[[Water Budget Diagrams]]
A: Water Surplus
-Precipitation is greater thanpotential evapotranspiration
- Soil Water store is full
B: Soil Water Utilisation
- p.EVT greater than precipitation
- water store used up by plants
D: Soil Moisture Deficiency
- Soil Moisture store used up
- precipitation absorbed: no run off
- river levels fall/dry up
E: Soil Moisture Recharge
- water stores start to fill
F: Field Capacity reached
- ground water stores used
Geography [[Weather and Climate]] 4.2
[[4.2 Why do seasonal variaty o f climates occur?]]
Equinox: 12 hours of daylight and 12 hours of darkness which occurs when the sun is directly above the equator.
Position of the Sun::
22nd December = Over Tropic of Capricorn = winter solstice
21st March = Over Equator = Vernal Equinox
21st June = Over Tropic of Cancer = Summer Solstice
23rd Spetmeber - Over Equator = Winter Equinox
The Tropicsheya: 231/3 N and 231/3 S
* Area inbetween the tropics of Cancer and Capricorn where at at least one occasion throughout the year the sun will be directly overhead.
* The closer the place to the tropic of cancer or capricorn, the closer together the times the sun is overhead.
- As the sun moved north, so do the pressure belts as they are due to the suns heating of the air
- sun moves through 47 degrees of latitude each year
Pressure belts move 5 degrees north and south of their central locations.
In June, the pressure belts have moved north
In December, the pressure belts have moved south
- The weather systems move with the pressure belts.
Geography [[Weather and Climate]] 4.2
[[4.2 Why do seasonal variations of climate occur?]]
The theoretical model of air rotation based on a non-rotating earth:
The actual model of air rotation on a rotating earth:
The ITCZ at the equator:
Pressure, Rainfall and frontal zones:
Tri-cellular Model of Air movement:
Geography [[Weather and Climate]] 4.2
[[4.2 Why do seasonal variations of climate occur?]]
Coriolis force = the effect that the rotationm of the earth has on any moving body.
*Northen Heisphere - air is deflected to the right by the coriolis force
*Southern hemisphere - air is deflected to the left by the coriolis force
*Where the winds meet at the equator they are rising, due to heat and spiral effect
* Air sinks and some heads back to the poles and some heads to equator
Front = the meeting of two types of air. Eg, the meeting of the warm tropical air and the cold polar air at the polar front. Here, the warm air rises up over the cold air.
Convergence = two lots of air coming together and rising leading to low pressure
Divergence = two lots of air meeting and sinking leading to high pressure.
Intertropical Convergence Zone (ITCZ): area of converging air at the equator
Antucyclones = areas of high pressures
Low Pressure formed by sinking air gives wet weather and leads to depressions
High pressure formed by rising air gives dry weather and leads to anticyclones
* Pressure belts are effected by the distribution of land and sea due to the ability of land/sea to warm up/cool down
Geography [[Weather and Climate]] 4.0
[[4.0 Why do Weather and Climate present a global challenge?]]
Climate = the longer term behaviour and character if the atmosphere
* Polar *Tropical * Equable *Means *Temperate *Seasonability * Extreme *Averages
Weather = the day to day events and short term changes
*Temperatures *Humidity *Rain *Snow *Cool *Clouds *Sunshine *Cold *Gale *Wind *Sleet
Areas of our life dependent on the weather or affected by the weather
* Sailors/Shipping
- short term - wind
*Agriculture
- Short Term = storms
- Long term = seasonality
*Tourism
- short term
*Hospitals/Health service
- short term - precipitation and temperature
*Emergancy Services
- short term - ice, fires, flash floods, gales etc
*Manufacturing
- long term
*local/national authorities
- short/long term = decision making
Climate = the longer term behaviour and character if the atmosphere
* Polar *Tropical * Equable *Means *Temperate *Seasonability * Extreme *Averages
Weather = the day to day events and short term changes
*Temperatures *Humidity *Rain *Snow *Cool *Clouds *Sunshine *Cold *Gale *Wind *Sleet
Areas of our life dependent on the weather or affected by the weather
* Sailors/Shipping
- short term - wind
*Agriculture
- Short Term = storms
- Long term = seasonality
*Tourism
- short term
*Hospitals/Health service
- short term - precipitation and temperature
*Emergancy Services
- short term - ice, fires, flash floods, gales etc
*Manufacturing
- long term
*local/national authorities
- short/long term = decision making
Thursday, 31 May 2007
Chemistry [[Module Three]] Alchols: Elimination
- Alcohols with H atom on carbon next to OH group can be dehydrated to alkenes
- 180 degrees
- sulphuric or conc. phosphoric catalyst.
- Mechanism = Elimination
- Reaction occurs in three steps:
1) Protonation (H+ added to OH group)
2) Loss of water (produces an carbocation)
3) Loss of a proton (produces alkene)

- 180 degrees
- sulphuric or conc. phosphoric catalyst.
- Mechanism = Elimination
- Reaction occurs in three steps:
1) Protonation (H+ added to OH group)
2) Loss of water (produces an carbocation)
3) Loss of a proton (produces alkene)

Chemistry [[Module Three]] Alcohols: Classifying and Reactions
- alcohols classified as primary, secondary or tertiary
- depends on which the carbon the OH group is attatched to
- Many reactions are the same, regardless of where the OH group is
- but three types of alchol differ when oxidised.
[[Oxidation of Alchol]]
- acidified potassium dichromate
- colour change from Orange to Green as Dichromate ions are reduced - test to distinguish primary and secondary from tertiary alcohols.
[Primary Alcohol]
- oxidised to Aldehydes
- colour change from orange to green
- Aldehyde still has H atom and so can be oxidised further
- Further oxidised to Carboxylic Acid
- To stop this happening, remove the aldehyde as it is produced using a condensor
- To get Carboxylic acid, prevent the product from escaping and heat under reflux
[Secondary Alcohol]
- oxidised to Ketones
- No further oxidation
- colour change from orange to green
[Tertiary Alcohols]
- no oxidation
- no colour change
[[Distinguishing bewteen Aldehydes and Ketones]]
- test for secondary/primary alchols
- use of mild oxidising agent
[[Fehlings Solution]]
- colour change from Blue to red in the presence of Aldehydes
- Not oxidised by Ketones
- Copper (II) Complex reduced to Copper (I)
[[Tollens Reagent]]
- Silver Mirror formed when warmed in the presence of Aldehydes
- Not oxidised by Ketones
- [Ag (NH302]+ reduced to Ag
Chemistry [[Module Three]] Alcohols: Ethanol Production
Alcohols = homologous series with general formula CnH2n+1OH
[[Ethanol Production]]
[Fermentation]
- living yeast
- converts sugars into ethanol and carbon dioxide
C6H12O6 --yeast--> 2C2H5OH + 2CO2
- slow at low temps
- at high temps enzymes are denatured
- compromise temp of 35 degrees
[Direct Hydration]
- produced industrially
- steam
-phosphoric acid catalyst
- pressure = 6.5x103
- temp = 300 degrees
C2H4 +H20 <===> C2H5OH
- currently preferred method of ethanol production in UK
- ethene is raw material
[Comparing]
Hydration:
* Fast reaction rate
* Pure product
* Finite raw material
* Continuous (cheap) process
* Expensive Equipment
Fermentation
* Slow reaction rate
* Impure product
* Infinite raw material
* Batch (expensive, manpower) process
* Cheap Equipment
[[Ethanol Production]]
[Fermentation]
- living yeast
- converts sugars into ethanol and carbon dioxide
C6H12O6 --yeast--> 2C2H5OH + 2CO2
- slow at low temps
- at high temps enzymes are denatured
- compromise temp of 35 degrees
[Direct Hydration]
- produced industrially
- steam
-phosphoric acid catalyst
- pressure = 6.5x103
- temp = 300 degrees
C2H4 +H20 <===> C2H5OH
- currently preferred method of ethanol production in UK
- ethene is raw material
[Comparing]
Hydration:
* Fast reaction rate
* Pure product
* Finite raw material
* Continuous (cheap) process
* Expensive Equipment
Fermentation
* Slow reaction rate
* Impure product
* Infinite raw material
* Batch (expensive, manpower) process
* Cheap Equipment
Chemistry [[Module Three]] Haloalkanes: Elimination
- Hydroxide ion can also act as a base
- which allows elimination to take place
- Hydrogen and Bromide elimated from haloalkane
- Forms an alkene
Mechanism:

[[Importance of Substitution and Elimination]]
1) Structure of Haloalkane
- primary haloalkanes give predominantly substitution reactions
- tertiary alkanes favour eliminatation
- secondary do both
2) Base Strength of the nucleophile
- liklihood increases as base strength on nucleophile increases
3) Reaction Conditions
- higher temps = greater chance of elmination
- Elimination = hot, ethanolic conditions
- Substitution = warm, aqueous conditions.
- which allows elimination to take place
- Hydrogen and Bromide elimated from haloalkane
- Forms an alkene
Mechanism:

[[Importance of Substitution and Elimination]]
1) Structure of Haloalkane
- primary haloalkanes give predominantly substitution reactions
- tertiary alkanes favour eliminatation
- secondary do both
2) Base Strength of the nucleophile
- liklihood increases as base strength on nucleophile increases
3) Reaction Conditions
- higher temps = greater chance of elmination
- Elimination = hot, ethanolic conditions
- Substitution = warm, aqueous conditions.
Chemistry [[Module Three]] Haloalkanes: Nucleophilic Substitution
[[Nucleophilic Substitution]]
Haloalkanes: homologous series of compunds with general formula CnH2n+1X where X represents F, Cl, Br and I.
- Halogens are electronegative
- Carbon Halogen bonds are polar
- The electrons in C-X bond are attracted to the halogen atom which gains a slight negative charge
- Therefore the Carbon has a slightly positve charge
- The slightly positive carbon is suceptable from attack by nucleophil
Nucleophile: And electron pair donor
- Nucleophilic attack occurs and the carbon halogen bond breaks, releasing a halide. The halide is replaced by the nucleophile.
- rate of reaction influenced by strength of C-X bond
- C-F bond is very polar but bond is very strong so Flouroalkanes are unreactive
- Chloroalkanes also react slowley
- Bromoalkanes react at a reasonable rate
[[Nucleophilic Substitution Reactions]]
[1. Hydroxide Ions]
- haloalkanes warned with aqueous sodium or potassium hydroxide
- forms alcohols
[2. Cyanide Ions]
- haloalkanes warmed with aqueous/alcholic solution of KCN
- nitriles formed
- carbon chain extended
[3. Ammonia]
- warmed with excess of ammonia
- sealed container
- Primary Amines
- excess of ammonia minimises chance of further reaction.

Chemistry [[Module Three]] Epoxyethane
- more commonly known as ethylene oxide
- highly reactive
- manufactured on large scale
- used in synthesis of important products
* Ethane 1,2-diol
* non-ionic surfactants
[[Production]]
- produced commercially by direct partial oxidation of ethene
- using oxygen/air
- silver based catalyst
- care needed in making and handling
- product = colourless gas
- product = flammable and explosive
- is toxic and may cause respiratory system irritation and neurological effects
- gas is excellent sterilising agent agaisnt bacteria.
[[Reactions]]
-three membered ring makes epoxyethane very reactive towards nucleophiles
Nucleophiles = An electron Pair Donor
-Exothermic reactions with nucleophiles cause the ring to open
- primary products contain 2-hydroxyethyl groups.
[Reaction With Water]
- half epoxyethane commercial produced is converted to ethane 1,2-diol by reaction with water
- Exothermic reaction
- Slow at room temperature
- acid catalyst used
- In industry = epoxyethane treated with ten-fold molar excess of water,, 60 degrees and sulphuric acid.
- Resulting solution is condensed by evapouration and fractional distilation
- yield is 90%
- other product = HOCH2CH2OCH2CH2OH
Wednesday, 30 May 2007
Chemistry [[Module Three]] Alkenes: Reactions
[[Hydrogenation]]

2) Hydrogen Bromide

NICKEL CAT
150 degrees
150 degrees
- uses = production of margerine
[[Electrophilic Addition]]
Electrophile: positive ions (or electrodeficient atoms) that act as eclectron pair acceptors and seek electron rich sites.
1) Bromine Water
- used as a test for alkenes since alkenes decolourise bromine water and alkanes do not

2) Hydrogen Bromide
3) Sulphuric Acid
[[To unsymmetrical Alkenes]]
If an alkene is unsymetrical, then electrophilic addition can produce two possible compounds.
The one that is more likley to occur is the one which forms the more stable carbocation
Tertiary Carbocations are more stable than Secondary carbocations which are more stable than primary carbocations
[[Direct Hydration of Ethene]]
- steam
- 300 degrees
- 6,5 x 103 KPa
H3PO4 cat

Chemistry [[Module Three]] Alkenes: Structure and Bonding
[[Structure and Bonding]]
Homologous series of hydrocarbons with general formula CnH12
Contain 2 hydrogens fewer than parent alkanes
Said to be unsaturated
Contain double bond which is an area of high electron density and the reason for the high reactivity of alkenes.
Homologous series of hydrocarbons with general formula CnH12
Contain 2 hydrogens fewer than parent alkanes
Said to be unsaturated
Contain double bond which is an area of high electron density and the reason for the high reactivity of alkenes.
Chemistry [[Module Three]] Alkanes: Chlorination
[[Chlorination]]
Alkanes do not react with chlorine at room temp but in the presence of UV light they react explosivly.
Process = Free Radical Substitution
-occurs in several steps
1) Inititaion
Cl2 ---> 2Cl*
The UV light provides activation energy by splitting a cholrine molecule into 2 chlorine free radicals.
This occurs first because Cl-Cl bond is weaker than the C-H bond in alkane.
2) Propagation
Cl* + CH4 --> CH3* + HCl
CH3* + Cl2 ---? CH3Cl + Cl*
A radical is used and a radical is formed, so it leads to a chain reaction.
Each step is exothermic
Overall equation: CH4 + Cl2 ---> CH3Cl + HCl
3) Termination
- two radicals combine to form a stable molecule and sequence of reations stop. Unpaired electrons form covalent bond.
Cl* + CH3* --> CH3Cl
CH3* + CH3* --> CH3CH3
Further Sunstitiution
-can occur to form
CH2Cl* --> CH2Cl2 and CHCl3
further substitution can be reduced if an excess of methane is used.
Alkanes do not react with chlorine at room temp but in the presence of UV light they react explosivly.
Process = Free Radical Substitution
-occurs in several steps
1) Inititaion
Cl2 ---> 2Cl*
The UV light provides activation energy by splitting a cholrine molecule into 2 chlorine free radicals.
This occurs first because Cl-Cl bond is weaker than the C-H bond in alkane.
2) Propagation
Cl* + CH4 --> CH3* + HCl
CH3* + Cl2 ---? CH3Cl + Cl*
A radical is used and a radical is formed, so it leads to a chain reaction.
Each step is exothermic
Overall equation: CH4 + Cl2 ---> CH3Cl + HCl
3) Termination
- two radicals combine to form a stable molecule and sequence of reations stop. Unpaired electrons form covalent bond.
Cl* + CH3* --> CH3Cl
CH3* + CH3* --> CH3CH3
Further Sunstitiution
-can occur to form
CH2Cl* --> CH2Cl2 and CHCl3
further substitution can be reduced if an excess of methane is used.
Chemistry [[Module Three]] Petroleum: Combustion
[[Combustion of Petroleum Fractions]]
Fractions obtained from petroleum are used as fuels
Hydrocarbons burn easily in air with exothermic reactions
Sulphur containing impurities occur with hydrocarbens and when these burn they produce oxides of sulphur which are toxic and dissolve in water producing acid rain.
[[Combustion of alkanes]]
Alkanes burn easily in air/Oxygen with very exothermic reactions
[Complete Combustion]
-Presence of sufficient oxygen
-CO2 and H2O formed
- As carbon chain increases, more oxygen is needed and more energy released
CH4 +2O2 --> C02 +H2O
C4H10 +61/2O2 ---> 4CO2 +5H2O
[[Incomplete Combustion]]
- presence of insufficient oxygen
- water is formed
- carbon monoxide or carbon is formed
- carbon monoxide = hazard = can lead to accidental death by CO poisening
[[Internal Combustion Engines]]
CO also formed by incomplete combustion of petrol vapour in car engine
Other pollutants formed - nitrous oxides mainly
Nitrous oxides formed when petrol/air mix is sparked and explodes -> provides enough energy for nitrogen to react woth oxygen
N2 +O2 --> 2NO
upon cooling it reacts with oxygen
2NO + O2 ---> 2NO2
and then with water and more oxygen
4NO2 + 2H2O + 02 ---> 4HNO3 = acid rain
[[Catalytic Converters]]
-help to remove pollutants from car wxhausts
- cermatic honeycomb coveredwith metals such as platinum, palladium and rhodiun
- These catalyse reactions between pollutants and remove 90% harmful gases
2CO + 2NO --> 2CO2 +N2
Pollutants become - CO2, N2 and H2O which are harmless
Fractions obtained from petroleum are used as fuels
Hydrocarbons burn easily in air with exothermic reactions
Sulphur containing impurities occur with hydrocarbens and when these burn they produce oxides of sulphur which are toxic and dissolve in water producing acid rain.
[[Combustion of alkanes]]
Alkanes burn easily in air/Oxygen with very exothermic reactions
[Complete Combustion]
-Presence of sufficient oxygen
-CO2 and H2O formed
- As carbon chain increases, more oxygen is needed and more energy released
CH4 +2O2 --> C02 +H2O
C4H10 +61/2O2 ---> 4CO2 +5H2O
[[Incomplete Combustion]]
- presence of insufficient oxygen
- water is formed
- carbon monoxide or carbon is formed
- carbon monoxide = hazard = can lead to accidental death by CO poisening
[[Internal Combustion Engines]]
CO also formed by incomplete combustion of petrol vapour in car engine
Other pollutants formed - nitrous oxides mainly
Nitrous oxides formed when petrol/air mix is sparked and explodes -> provides enough energy for nitrogen to react woth oxygen
N2 +O2 --> 2NO
upon cooling it reacts with oxygen
2NO + O2 ---> 2NO2
and then with water and more oxygen
4NO2 + 2H2O + 02 ---> 4HNO3 = acid rain
[[Catalytic Converters]]
-help to remove pollutants from car wxhausts
- cermatic honeycomb coveredwith metals such as platinum, palladium and rhodiun
- These catalyse reactions between pollutants and remove 90% harmful gases
2CO + 2NO --> 2CO2 +N2
Pollutants become - CO2, N2 and H2O which are harmless
Chemistry [[Module Three]] Petroleum: Cracking
[[Cracking]]
Involves breaking C-C and C-H bonds
Higher Mr Alkanes -------> Smaller Mr Alkanes + Alkenes
Molecules can break up several different ways to form a micture of products, which is then seperated by fractional distiliation.
[[Thermal Cracking]]
Results in formation of a high proportion of alkenes
energy required for bond breaking provieded by heat
Temps range from 400 to 900 degrees at pressures of 7000 KPa
At lower end of temperature range, carbon chains break up near to middle of chain
At higher end, carbon chains break up towards the end keading to a greater % low Mr alkenes
The length of exposure has to be short, to avoid decomposition
1) Initiated by homolytic fission of C-C bond to form to alkyl radicals
2) Each alkyl free radical can abstract a hydrogen molecule from an alkane to produce a different alkyl radical and a shorter alkane:
CH3(CH2)6CH3 ---> CH3CH2CH2CH2CH2* + *CH2CH2CH3
CH3(CH2)6CH3 + CH3CH2CH2CH2CH2* ---> CH3(CH2)5C*HCH3
Radical: Species which results from homolytic fission of a covalent bond. They contain an odd n umber of electrons with one unpaired electron. Written as a dot (* on this blog)
[[Catalytic Cracking]]
Involves the use of ZEOLITE catalysts
Slight excess of pressure
Temperature = 450 degrees
Large alkanes converted to branched chain alkanes, cycloalkanes and aromatic hydrocarbons
C14H30 -------> C8H18 + C6H12
Alkene proportion is small
Catalytic cracking = used for producing motor fuels
Branched Chain burn more smoothly than unbranched alkanes
Branched chain alkanes used as fuel to prevent the problem of knocking when the fuel air mixture tries to ignite before spark is produced.
In catalytic cracking, the catalyst acts as a lewis acid and the method involves the formation of carbocations
Involves breaking C-C and C-H bonds
Higher Mr Alkanes -------> Smaller Mr Alkanes + Alkenes
Molecules can break up several different ways to form a micture of products, which is then seperated by fractional distiliation.
[[Thermal Cracking]]
Results in formation of a high proportion of alkenes
energy required for bond breaking provieded by heat
Temps range from 400 to 900 degrees at pressures of 7000 KPa
At lower end of temperature range, carbon chains break up near to middle of chain
At higher end, carbon chains break up towards the end keading to a greater % low Mr alkenes
The length of exposure has to be short, to avoid decomposition
1) Initiated by homolytic fission of C-C bond to form to alkyl radicals
2) Each alkyl free radical can abstract a hydrogen molecule from an alkane to produce a different alkyl radical and a shorter alkane:
CH3(CH2)6CH3 ---> CH3CH2CH2CH2CH2* + *CH2CH2CH3
CH3(CH2)6CH3 + CH3CH2CH2CH2CH2* ---> CH3(CH2)5C*HCH3
Radical: Species which results from homolytic fission of a covalent bond. They contain an odd n umber of electrons with one unpaired electron. Written as a dot (* on this blog)
[[Catalytic Cracking]]
Involves the use of ZEOLITE catalysts
Slight excess of pressure
Temperature = 450 degrees
Large alkanes converted to branched chain alkanes, cycloalkanes and aromatic hydrocarbons
C14H30 -------> C8H18 + C6H12
Alkene proportion is small
Catalytic cracking = used for producing motor fuels
Branched Chain burn more smoothly than unbranched alkanes
Branched chain alkanes used as fuel to prevent the problem of knocking when the fuel air mixture tries to ignite before spark is produced.
In catalytic cracking, the catalyst acts as a lewis acid and the method involves the formation of carbocations
Chemistry [[Module Three]] Petroleum: Fractional Distilation
[[Petroleum]]
Complex micture of hydrocarbens (mainly alkanes)
Derived from remains of sea creatures and plants which died and sank to the sea floor millions of years ago.
Susequent deposits compressed the material
High pressures, high temperatures and lack of air converted it to oil and gass.
[[Alkanes]]
Homologous series of saturdated hydrocarbens with the general forumula CnH2n+2
Boiling points increase with length of carbons as van der waals forces between the molecules increase
Increase of boiling points allows for seperation of crude oil by fractional distilation
[[Fractional Distilation]]
Seperation of the mixture of alkanes in crude oil into less complicated mixtures (or fractions)
1) Crude Oil Heated
2) Vapour/Mixture passed into a tower
3) Temperature gradient in tower means that it is cooler at the top than it is at the bottom
4) The temperature gradient seperates the mixture into fractions depending on the boiling points of the compounds present
The hydrocarbens with low boiling points reach the top
The other condense in trays at different levels along the tube and are drawn off
The residue still contains useful materials, sich as lubricting oils and waxes
These boil at above 350 degrees at atmospheric pressure
To avoid such high temps (At which other useful products would decompose) the residue is distilled under reduced pressure duing vacuum distillation.
In vacuum distilation, the remaining hydrocarbens can be distilled at lower temperatures without decomposing.
The amount of products produced from fractional distilation does not meet the demand, so to solve this, longer chain alkanes are broken up in a process called Cracking.
Complex micture of hydrocarbens (mainly alkanes)
Derived from remains of sea creatures and plants which died and sank to the sea floor millions of years ago.
Susequent deposits compressed the material
High pressures, high temperatures and lack of air converted it to oil and gass.
[[Alkanes]]
Homologous series of saturdated hydrocarbens with the general forumula CnH2n+2
Boiling points increase with length of carbons as van der waals forces between the molecules increase
Increase of boiling points allows for seperation of crude oil by fractional distilation
[[Fractional Distilation]]
Seperation of the mixture of alkanes in crude oil into less complicated mixtures (or fractions)
1) Crude Oil Heated
2) Vapour/Mixture passed into a tower
3) Temperature gradient in tower means that it is cooler at the top than it is at the bottom
4) The temperature gradient seperates the mixture into fractions depending on the boiling points of the compounds present
The hydrocarbens with low boiling points reach the top
The other condense in trays at different levels along the tube and are drawn off
The residue still contains useful materials, sich as lubricting oils and waxes
These boil at above 350 degrees at atmospheric pressure
To avoid such high temps (At which other useful products would decompose) the residue is distilled under reduced pressure duing vacuum distillation.
In vacuum distilation, the remaining hydrocarbens can be distilled at lower temperatures without decomposing.
The amount of products produced from fractional distilation does not meet the demand, so to solve this, longer chain alkanes are broken up in a process called Cracking.
Chemistry [[Module Three]] Nomenclature and Isomerism
[[Isomerism]]
Occurs when molecules with the same molecular formula have their atoms aranged in different ways. It is sub divided.
[[Structural Isomerism]]
COMPOUNDS WITH THE SAME MOLECULAR FORMULAE BUT WITH DIFFERENT STRUCTURES
[Chain Isomerism]
occurs when there are two or more ways of arranging the carbon skeleton of a compound. The isomers have similar chemical properties byr slightly different physical properties. Branched isomers have smaller volumes, weaker van der waals and threrefore lower boiling points.
Isomers of C5H12:

[Position Isomerism]
Isomers have the same carbon skeleton and the same functional group but the functional group is joined at different places on the carbon skeleton.
CH3CH2CH2BR = 1-bromopropane
CH3CHBrCH3 = 2-bromopropane
CH2=CHCH2CH3 = but-1-ene
CH3CH=CH2 = but-2-ene
They have similar chemical properties but the different positions cause differences in physical properties.
[Functional Group]
Isomers have different functional groups
they have different physical and chemical properties.
C3H6O:
CH3CH3CHO or CH3COCH3
propanal propanone
C3H6O2
CH3CH2COOH or CH3COOCH3 or HCOOCH2CH3
propanoic acid methyl ethanoate ethyl methanoate
[[Stereoisomerism]]
MOLECULES WHICH HAVE THE SAME STRUCTURAL FORMULA BUT THEIR BONDS ARE ARRANGED DIFFERENTLY IN SPACE
[Geometrical (Cis-Trans0 Isomerism]
Carbon carbon double bonds cannot rotate died to electron clouds above and below plane of the bond.
If there are two different groups at each end of the double bond then cis-trans isomerism results.
Cis: Two identical groups on the same side of the double bond
Trans: Two identical groups on oppersite sides of the double bond
Geometrical Isomerism is not possible when there are two identical groups on the same carbon.

Occurs when molecules with the same molecular formula have their atoms aranged in different ways. It is sub divided.
[[Structural Isomerism]]
COMPOUNDS WITH THE SAME MOLECULAR FORMULAE BUT WITH DIFFERENT STRUCTURES
[Chain Isomerism]
occurs when there are two or more ways of arranging the carbon skeleton of a compound. The isomers have similar chemical properties byr slightly different physical properties. Branched isomers have smaller volumes, weaker van der waals and threrefore lower boiling points.
Isomers of C5H12:

[Position Isomerism]
Isomers have the same carbon skeleton and the same functional group but the functional group is joined at different places on the carbon skeleton.
CH3CH2CH2BR = 1-bromopropane
CH3CHBrCH3 = 2-bromopropane
CH2=CHCH2CH3 = but-1-ene
CH3CH=CH2 = but-2-ene
They have similar chemical properties but the different positions cause differences in physical properties.
[Functional Group]
Isomers have different functional groups
they have different physical and chemical properties.
C3H6O:
CH3CH3CHO or CH3COCH3
propanal propanone
C3H6O2
CH3CH2COOH or CH3COOCH3 or HCOOCH2CH3
propanoic acid methyl ethanoate ethyl methanoate
[[Stereoisomerism]]
MOLECULES WHICH HAVE THE SAME STRUCTURAL FORMULA BUT THEIR BONDS ARE ARRANGED DIFFERENTLY IN SPACE
[Geometrical (Cis-Trans0 Isomerism]
Carbon carbon double bonds cannot rotate died to electron clouds above and below plane of the bond.
If there are two different groups at each end of the double bond then cis-trans isomerism results.
Cis: Two identical groups on the same side of the double bond
Trans: Two identical groups on oppersite sides of the double bond
Geometrical Isomerism is not possible when there are two identical groups on the same carbon.

Chemistry [[Module Three]] Nomencalture Naming Organic Compounds
[[Rules]]
1) Identify the length of the carbon chain, and name accordingly
Numbers of carbon in chain:
1 = methane = CH3
2 = ethane = Ch2CH3
3 = propane = CH2CH2CH3
4 = butane = CH2CH2CH2CH3
5 = pentane = CH2CH2CH2CH2CH3
6 = hexane = CH2CH2CH2CH2CH2CH3
2) Number the carbons so that any branches/functional groups fall on the lowest posible carbon
3) Identify branches/functional groups
List functional groups/branches in alphabetical order. This takes precedence over the numbers of the groups.
The Functional Groups:

1) Identify the length of the carbon chain, and name accordingly
Numbers of carbon in chain:
1 = methane = CH3
2 = ethane = Ch2CH3
3 = propane = CH2CH2CH3
4 = butane = CH2CH2CH2CH3
5 = pentane = CH2CH2CH2CH2CH3
6 = hexane = CH2CH2CH2CH2CH2CH3
2) Number the carbons so that any branches/functional groups fall on the lowest posible carbon
3) Identify branches/functional groups
List functional groups/branches in alphabetical order. This takes precedence over the numbers of the groups.
The Functional Groups:

Chemistry [[Module Three]] Nomenclature and Isomerism
[[Nomenclature]]
Empirical Formula: Simplest ratio of atoms of each element in a compound
Molecular Formula: Actual number of atoms of each element present in a compound.
To find emirical formula: Compoun X is found to contain 40.0% carbon, 6.7% Hudrogen and 53.3% Oxygen by mass.
% Mass 40.0 6.7 53.3
/Ar 40.0/12 = 3.33 6.7/1 = 6.7 53.3/16 = 3.33
/smallest 3.33/3.33 = 1 6.7/3.33 = 2 3.33/3.33 = 1
Simplest 1 2 1
ratio
Empircal Formula = CH2O
Finding the Molecular Formula - you need to know the mass of the compound.
Work out mass of empirical.
Equation = Molecular Formula = mass of molecular/mass of empirical x empircal formula.
Example = mass of empirical = 12 + 2 +16 = 30
mass of molecular = 60
60/30 = 2 x CH20 = C2H4O2
Isomers: Compounds with the same molecular formula, but in which the elements are arranged differently
Structural Isomers: Compounds with the same molecular formular but with different structures and represented by different structural formula
Structural Formula: shows the atoms present and all of the bonds between the atoms
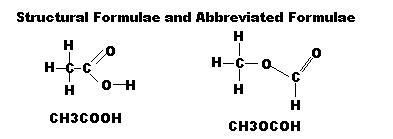
Functional Group: atom or group of atoms which, when present in different molecules, causes them to have simlar chemical properties
Homologous Series: A family of molecules which all contain the same functional group and an increasing number of carbons
CnH2n+2 = alkanes
CnH2n = alkenes and cyclic alkanes
CnH2n+1OH = alcohols
All members of the same homologous series have similar chemical properties.
Their physical properties gradually change as the length of the carbon chain increases. For example, boiling point of alkanes increases as number of carbons increase.
Empirical Formula: Simplest ratio of atoms of each element in a compound
Molecular Formula: Actual number of atoms of each element present in a compound.
To find emirical formula: Compoun X is found to contain 40.0% carbon, 6.7% Hudrogen and 53.3% Oxygen by mass.
% Mass 40.0 6.7 53.3
/Ar 40.0/12 = 3.33 6.7/1 = 6.7 53.3/16 = 3.33
/smallest 3.33/3.33 = 1 6.7/3.33 = 2 3.33/3.33 = 1
Simplest 1 2 1
ratio
Empircal Formula = CH2O
Finding the Molecular Formula - you need to know the mass of the compound.
Work out mass of empirical.
Equation = Molecular Formula = mass of molecular/mass of empirical x empircal formula.
Example = mass of empirical = 12 + 2 +16 = 30
mass of molecular = 60
60/30 = 2 x CH20 = C2H4O2
Isomers: Compounds with the same molecular formula, but in which the elements are arranged differently
Structural Isomers: Compounds with the same molecular formular but with different structures and represented by different structural formula
Structural Formula: shows the atoms present and all of the bonds between the atoms

The abbreviated Formulae are used in equations, but Molecular Formulae should not be used. This avoids confusion.
Functional Group: atom or group of atoms which, when present in different molecules, causes them to have simlar chemical properties
Homologous Series: A family of molecules which all contain the same functional group and an increasing number of carbons
CnH2n+2 = alkanes
CnH2n = alkenes and cyclic alkanes
CnH2n+1OH = alcohols
All members of the same homologous series have similar chemical properties.
Their physical properties gradually change as the length of the carbon chain increases. For example, boiling point of alkanes increases as number of carbons increase.
Wednesday, 23 May 2007
Chemsitry [[The A2 practical]] June 2006 Paper and Mark Scheme
3) Planning - Finding the order of a chemical reaction.
* State appropriate volume of gas - 20-25cm
* Use this volume to calculate the second calculation, in this case the volume of solution needed for dilution.
Apparatus:
* Appropriate container for reaction - any vessel with a gas outlet and a stopper
* Appropriate collection of gas - over water or by syringe
* Apparatus for measuring volume of solution - measuring cylinder, pipette, buerette etc
* Thermostatic control - waterbath
Method:
* measure out specified volume of solution
* Keep mixtures at 20 degrees
* Take volume readings at suitable time interviews (or measure time taken to collect specific volume of gas).
* Experiment with at leats 2 concentrations of gas
Results
* Mesasure volumes at regular untervals
* plot senisble graph of results of volume vs time
* Clear and correct explaination of rate from graph
* clear correct explanation of use of rate data to establish first order
Hazards
* Phenol = toxic/corrosiv = wash spillages with cold water
* Eye protection.
* Pipette filler
* State appropriate volume of gas - 20-25cm
* Use this volume to calculate the second calculation, in this case the volume of solution needed for dilution.
Apparatus:
* Appropriate container for reaction - any vessel with a gas outlet and a stopper
* Appropriate collection of gas - over water or by syringe
* Apparatus for measuring volume of solution - measuring cylinder, pipette, buerette etc
* Thermostatic control - waterbath
Method:
* measure out specified volume of solution
* Keep mixtures at 20 degrees
* Take volume readings at suitable time interviews (or measure time taken to collect specific volume of gas).
* Experiment with at leats 2 concentrations of gas
Results
* Mesasure volumes at regular untervals
* plot senisble graph of results of volume vs time
* Clear and correct explaination of rate from graph
* clear correct explanation of use of rate data to establish first order
Hazards
* Phenol = toxic/corrosiv = wash spillages with cold water
* Eye protection.
* Pipette filler
Chemistry [[The A2 Practical Exam]] Preparation of Aspirin
[[The preparation of 2-hydroxybenzoic acid]]
1. Set up appartus to heat 30cm3 reaction mixture in a waterbath with the use of a condensor to prevent the loss of product
2. 2g Oil of wintegreen into flask
3. Add 25cm3 of 2 molar sodium hydroxide and antibumping granules
4. Heat over boiling water bath for 30 minutes
5. Pour mixture into beaker surrounded by ice and water
6. Add HCl drop by drop until solution is slightly acidic, stirring at all times
7. Filter product using buchner funnel
8. Wash product with ice water and transfer to weight watch glass
7. Leave to dry over night
[[The preparation of Aspirin]]
1. Use 1g of 2-hydroxybenzoic acid and weigh accuratly
2. Put into pear shaped flask
3. Add 2cm3 ethanoic anhydride and 8 drops of concentrated phosphoric acid. Put condensor on the flask.
4. Warm mixture in hot bath in fume cupboard until all the mixture has disolved and for a further 5 minutes
5. Add 5cm3 of cold water
6. Stand in bath of iced water until ppt is complete - may be necessary to stir vigorously to start ppt process
7. Filter off product using buchner funnel
8. Wash with cold water and transfer to a weighted watch glass
9. Leave to dry over night
10. Weigh product.
[[Recrystallisation]]
1. Dissolve sample in minimum quantity of hot solvent
2. Filter the hot solution using buchner funnel
3. Allow solution to cool slowley
4. Filter cool solution using buchner funnel
5. Wash residue with cold solvent
6. Dry filtrate and Weigh.
1. Set up appartus to heat 30cm3 reaction mixture in a waterbath with the use of a condensor to prevent the loss of product
2. 2g Oil of wintegreen into flask
3. Add 25cm3 of 2 molar sodium hydroxide and antibumping granules
4. Heat over boiling water bath for 30 minutes
5. Pour mixture into beaker surrounded by ice and water
6. Add HCl drop by drop until solution is slightly acidic, stirring at all times
7. Filter product using buchner funnel
8. Wash product with ice water and transfer to weight watch glass
7. Leave to dry over night
[[The preparation of Aspirin]]
1. Use 1g of 2-hydroxybenzoic acid and weigh accuratly
2. Put into pear shaped flask
3. Add 2cm3 ethanoic anhydride and 8 drops of concentrated phosphoric acid. Put condensor on the flask.
4. Warm mixture in hot bath in fume cupboard until all the mixture has disolved and for a further 5 minutes
5. Add 5cm3 of cold water
6. Stand in bath of iced water until ppt is complete - may be necessary to stir vigorously to start ppt process
7. Filter off product using buchner funnel
8. Wash with cold water and transfer to a weighted watch glass
9. Leave to dry over night
10. Weigh product.
[[Recrystallisation]]
1. Dissolve sample in minimum quantity of hot solvent
2. Filter the hot solution using buchner funnel
3. Allow solution to cool slowley
4. Filter cool solution using buchner funnel
5. Wash residue with cold solvent
6. Dry filtrate and Weigh.
Chemistry [[The A2 Practical Exam]] Redox Titrations
The two most widley used oxidising agents and their reduction equation:
Potassium Manganate (VII)
MnO4- + 8H+ + 5 e- ---> Mn2+ + 4H2O
Potassium Dichromate (VI)
Cr2O72- + 14H+ + 6e- ---> 2Cr3+ + 7H20
[[Analysis of Iron Tablets]]
[Introduction]
Iron Tablets from the pharmacist contain anhydrous iron (11) sulphate, cheap and soluble form of iron, plus unreactive binders. Iron tablets are taken to boost Fe2+ concentration in the blood of anaemic people.
Each tablet can be dissolved in dilute sulphuric acid. Assuming that all the iron in the tablet is Fe2+ and that it is all dissolved, it is possible toe estimate the percentage iron (ii) sulphate content of each tablet by titration agaisnt standardised potassium manganate VII.
[Proceedure]
1) Weigh out 8 iron t ablets and record the mass
2) Dissolve the tablets in about 100cm3 of 2M sulphuric acid
3) Outer coat will not dissolve so filter the substance
4) Pour filtrate into 250cm3 volumetric flask, add washings from conical flask and filterpaper. Make up to the mark with distilled water.
5) Pipettes 25cm3 into conical flask and add about 25cm3 of dilute sulphuric acid
6) Titrate agaisnt 0.020M potassium manganate VII. The end point is a colour change from colourless to permanant pink
7) Repeat until concordant results are obtained.
[Notes]
*Potassium Manganate (VII) in burette and acidified iron (II) solution in conical flask.
*Both oxidising agents work strongly in acidic conditions - H+ ions are needed for the reaction.
*Choice of acid is important - HCl cannot be used in manganate titrations because the Mn will oxidise the Cl- to Cl2. But HCL can be used in dichromate titrations since the dichromate ions i=s not strong enough to oxidise the Cl- ions. Weak acids (ethanoic) cannot be uised as they do not provide a high enough [H+]
*No indicator is used for managanate as the solution acts as its own indicator. The purple manganate (VII) is reduced to manganese (II) which is colourless. One drop of manganate (VII) will produce a permantly pale pink colour = endpoint.
* Potassium dichromate needs a indicator = barium N-phenolphenylamine-4-sulphonate. Endpoint = blue green to violet.
* H3PO4 is added to enhance the end point of potassium dichromate titrations.
[results]
Mass of iron tablets = 6.82g
Mean Titre Value = 24.55cm3
1) Find the moles of MnO4- = V/1000 x c = 24.55/1000 x 0.020 = 4.91 x 10-4
2) Molar ratio from equation = 1:5
3) Find moles of Fe2+ in 25cm3 = 4.91 x 10-4 x 5 = 2.45 x 10-4
4) Find moles of Fe2+ in 250cm3 = 2.45 x 10-4 x 10 = 2,45 x 10-3
5) Mr of FeS04 = 152
6) Mass of FeS04 in tablets = m = n x mr = 2.45x10-3 x 152 = 3.373g
7) find % mass =
3.373/6.8 x 100 =
54.7%
Potassium Manganate (VII)
MnO4- + 8H+ + 5 e- ---> Mn2+ + 4H2O
Potassium Dichromate (VI)
Cr2O72- + 14H+ + 6e- ---> 2Cr3+ + 7H20
[[Analysis of Iron Tablets]]
[Introduction]
Iron Tablets from the pharmacist contain anhydrous iron (11) sulphate, cheap and soluble form of iron, plus unreactive binders. Iron tablets are taken to boost Fe2+ concentration in the blood of anaemic people.
Each tablet can be dissolved in dilute sulphuric acid. Assuming that all the iron in the tablet is Fe2+ and that it is all dissolved, it is possible toe estimate the percentage iron (ii) sulphate content of each tablet by titration agaisnt standardised potassium manganate VII.
[Proceedure]
1) Weigh out 8 iron t ablets and record the mass
2) Dissolve the tablets in about 100cm3 of 2M sulphuric acid
3) Outer coat will not dissolve so filter the substance
4) Pour filtrate into 250cm3 volumetric flask, add washings from conical flask and filterpaper. Make up to the mark with distilled water.
5) Pipettes 25cm3 into conical flask and add about 25cm3 of dilute sulphuric acid
6) Titrate agaisnt 0.020M potassium manganate VII. The end point is a colour change from colourless to permanant pink
7) Repeat until concordant results are obtained.
[Notes]
*Potassium Manganate (VII) in burette and acidified iron (II) solution in conical flask.
*Both oxidising agents work strongly in acidic conditions - H+ ions are needed for the reaction.
*Choice of acid is important - HCl cannot be used in manganate titrations because the Mn will oxidise the Cl- to Cl2. But HCL can be used in dichromate titrations since the dichromate ions i=s not strong enough to oxidise the Cl- ions. Weak acids (ethanoic) cannot be uised as they do not provide a high enough [H+]
*No indicator is used for managanate as the solution acts as its own indicator. The purple manganate (VII) is reduced to manganese (II) which is colourless. One drop of manganate (VII) will produce a permantly pale pink colour = endpoint.
* Potassium dichromate needs a indicator = barium N-phenolphenylamine-4-sulphonate. Endpoint = blue green to violet.
* H3PO4 is added to enhance the end point of potassium dichromate titrations.
[results]
Mass of iron tablets = 6.82g
Mean Titre Value = 24.55cm3
1) Find the moles of MnO4- = V/1000 x c = 24.55/1000 x 0.020 = 4.91 x 10-4
2) Molar ratio from equation = 1:5
3) Find moles of Fe2+ in 25cm3 = 4.91 x 10-4 x 5 = 2.45 x 10-4
4) Find moles of Fe2+ in 250cm3 = 2.45 x 10-4 x 10 = 2,45 x 10-3
5) Mr of FeS04 = 152
6) Mass of FeS04 in tablets = m = n x mr = 2.45x10-3 x 152 = 3.373g
7) find % mass =
3.373/6.8 x 100 =
54.7%
Sunday, 13 May 2007
Chemistry [[The Practical Exam]] Planning Technique
[[Working Out the Volume to Use]]
* Pick suitable volumes for the equipment that you are using and justify any measurements stated.
E.g,
CuO(s) + H2S04(aq) ---> CuSO4 + H2O
* reaction done in a 100ml beaker
* Saftey issues
* Use 25cm3 of H2SO4
- caluculate mass of CuO to use, using n = m/mr and n = v/1000 x c
= 2grams
In excess?
* think about how to seperate the final products
H2SO4 and CuSO4 cannot be seperated easily
Cu0 and CuSO4 can be seperated by filtering so double the value
= 4g of CuSO4
[[Measuring a Temperature]]
*change the concentraition to create a suitable change in temp
* Pick suitable volumes for the equipment that you are using and justify any measurements stated.
E.g,
CuO(s) + H2S04(aq) ---> CuSO4 + H2O
* reaction done in a 100ml beaker
* Saftey issues
* Use 25cm3 of H2SO4
- caluculate mass of CuO to use, using n = m/mr and n = v/1000 x c
= 2grams
In excess?
* think about how to seperate the final products
H2SO4 and CuSO4 cannot be seperated easily
Cu0 and CuSO4 can be seperated by filtering so double the value
= 4g of CuSO4
[[Measuring a Temperature]]
*change the concentraition to create a suitable change in temp
Chemistry [[The Practical Exam]] How to Carry Out a Titration
[[Making A Standar Solution]]
A solution of a known concentration
There are two ways of making a standard solution: Solid and Liquid
[[Liquid]]
1) Use an appropriate sized volumetric flask (250ml)
2) Clean a 25ml pipette with the solution to be diluted using a pipette filler
3) Fill the pipette up to the mark with the solution to be diluted. Read from the bottom of the meniscus
4) Allow solution to drain under gravity into the volumetric flask and touch surface of solution with pipette
5) Make up to the mark with distilled water
6) Shake to ensure contents are mixed.
[[Solid]]
1) Work out the mass of solid needed:
To prepare 250cm3 of 0.1M NaOH solution from NaOH solid:
* Work out the nuumber of moles.
n = v/1000 x c so n = 250/1000 x 0.1 so n = 0.025mols
*Work out the mass
n = m/mr so m = n x mr sp m = 0.025 x 40 so m = 1.00g
2) Mass is weighed out on an accurate mass balance
3) Carefully transfer into volumetric flask
4) Wash weighing boat with distilled water and transfer washings into volumetric flask
5) Make up to the mark with distilled water
6) Shake flask to ensure that contents are mized.
[[The Titration]]
* Acid/Alkali titrations = acid in burette
* Use appropriate size burette and flask
2) Rinse out pipette
3) Fill pipette using pipette filler, up to the mark, and then allow contents to drain under gravity into conical flask.
4) Touch pipette tip to surface of solution to allow for capillary action
5) Add a few drops of appropriate inditcator
6) Rinse out burette
7) Fill burette using funnel ensuring that tap is closed
8) remove air space between tap and tip of burette and remove funnel
9) Read initial volume at eye level of solution in the burette
10) Carry out a rough titration - allow to drain and shake to mix acid with alkali
11) When colour of indicaor permanatly changes, stop adding from burette -> record volume at eye level
12) Volume is always quoted to 2 decimal places, the first one always '0' or '5'
13) Repeat using fresh solution, HOWEVER
i) Towards end point add solution drop by drop
ii) Rinse sides of conical flask with a little distilled water
14) Repeat again until at least two concordant values have been obtained.
[[The results]]
- All results should be recorded
- Average out the concordant values.
[[Health and Safety]]
- Acid and alkali are corrosive
- wear saftey glasses
- rinse spillages from skin immediatly
- mop up spillages on bench
- wear gloves for highly corrosive or toxic materials
[[Errors]]
- There are two types of error in a practical exam
* Measuring
* Procedural
% error in measuring = accuracy of measuring equipment/measurement made x 100
% difference = difference between actual and expected result/expected result x 100
A solution of a known concentration
There are two ways of making a standard solution: Solid and Liquid
[[Liquid]]
1) Use an appropriate sized volumetric flask (250ml)
2) Clean a 25ml pipette with the solution to be diluted using a pipette filler
3) Fill the pipette up to the mark with the solution to be diluted. Read from the bottom of the meniscus
4) Allow solution to drain under gravity into the volumetric flask and touch surface of solution with pipette
5) Make up to the mark with distilled water
6) Shake to ensure contents are mixed.
[[Solid]]
1) Work out the mass of solid needed:
To prepare 250cm3 of 0.1M NaOH solution from NaOH solid:
* Work out the nuumber of moles.
n = v/1000 x c so n = 250/1000 x 0.1 so n = 0.025mols
*Work out the mass
n = m/mr so m = n x mr sp m = 0.025 x 40 so m = 1.00g
2) Mass is weighed out on an accurate mass balance
3) Carefully transfer into volumetric flask
4) Wash weighing boat with distilled water and transfer washings into volumetric flask
5) Make up to the mark with distilled water
6) Shake flask to ensure that contents are mized.
[[The Titration]]
* Acid/Alkali titrations = acid in burette
* Use appropriate size burette and flask
2) Rinse out pipette
3) Fill pipette using pipette filler, up to the mark, and then allow contents to drain under gravity into conical flask.
4) Touch pipette tip to surface of solution to allow for capillary action
5) Add a few drops of appropriate inditcator
6) Rinse out burette
7) Fill burette using funnel ensuring that tap is closed
8) remove air space between tap and tip of burette and remove funnel
9) Read initial volume at eye level of solution in the burette
10) Carry out a rough titration - allow to drain and shake to mix acid with alkali
11) When colour of indicaor permanatly changes, stop adding from burette -> record volume at eye level
12) Volume is always quoted to 2 decimal places, the first one always '0' or '5'
13) Repeat using fresh solution, HOWEVER
i) Towards end point add solution drop by drop
ii) Rinse sides of conical flask with a little distilled water
14) Repeat again until at least two concordant values have been obtained.
[[The results]]
- All results should be recorded
- Average out the concordant values.
[[Health and Safety]]
- Acid and alkali are corrosive
- wear saftey glasses
- rinse spillages from skin immediatly
- mop up spillages on bench
- wear gloves for highly corrosive or toxic materials
[[Errors]]
- There are two types of error in a practical exam
* Measuring
* Procedural
% error in measuring = accuracy of measuring equipment/measurement made x 100
% difference = difference between actual and expected result/expected result x 100
Monday, 7 May 2007
Biology [[Control, Coordination and Homeostasis]] The Structure iof the Kidney
[[Structure of the Kidney]]
Kidneys recieves blood from a renal artery and returns it via a renal vein. Urine is carried from the kidney to the bladder via the ureter. From the bnladder, the uretha carries it to the outside of the body.
The kidney has three main areas.
* The whole kidney is covered by the capsule.
* Underneath the capsule lies the cortex
* The central area is called the medulla
* The area where the ureter joins is called the pelvis
Kidneys are made up of tiny tubes called nephrons
One end of the nephron is cup shaped, and is called a Bowmans capsule (or renal capsule) and these are located in the cortex of the kidney.
The tube then runs towards the center of the kidney, forming a twisted region called the proximal convoluted tuble. Then it runs in a long, hair pin loop called the loop of henle.
The tube runs back up into the cortx where it forms another twisted region called the distal convoluted tube
The tube joins a collecting duct, which leads down through the medulla and into the pelvis of the kidney, where it joins with the ureter.
Each renal capsule is supplied with blood by a branch of the renal artery - 'afferent arteriole' which splits into a glomerulus. These rejoin to form an 'efferent arteriole' and then links back of with other capillaries to go to the renal vein.
Kidneys recieves blood from a renal artery and returns it via a renal vein. Urine is carried from the kidney to the bladder via the ureter. From the bnladder, the uretha carries it to the outside of the body.
The kidney has three main areas.
* The whole kidney is covered by the capsule.
* Underneath the capsule lies the cortex
* The central area is called the medulla
* The area where the ureter joins is called the pelvis
Kidneys are made up of tiny tubes called nephrons
One end of the nephron is cup shaped, and is called a Bowmans capsule (or renal capsule) and these are located in the cortex of the kidney.
The tube then runs towards the center of the kidney, forming a twisted region called the proximal convoluted tuble. Then it runs in a long, hair pin loop called the loop of henle.
The tube runs back up into the cortx where it forms another twisted region called the distal convoluted tube
The tube joins a collecting duct, which leads down through the medulla and into the pelvis of the kidney, where it joins with the ureter.
Each renal capsule is supplied with blood by a branch of the renal artery - 'afferent arteriole' which splits into a glomerulus. These rejoin to form an 'efferent arteriole' and then links back of with other capillaries to go to the renal vein.
Biology [[Control, Coordination and Homeostasis]] Excretion
[[Excretion]]
Metabolic reactions occuring within the body produce unwanted substances.
Some are toxic
Excretion if the removal of these waste products.
The two products made in greater amounts than most in the human body are CO2 and Urea.
* CO2 is produced continuously by every cell in the body by aerobic respiration The waste CO2 is transported via blood to the alveoli where it is excreted into the air that we breath out.
*Urea is produced un only one organ in the body: The Liver
Its is produced from excess amino acides and is transported from the liver to the kidnesy, in solution in blood plasma.
The kidneys remove urea from the blood and excrete it dissolved in water as urine.
[[Deamination]]
More Protien is eaten than is needed, the excess cannot be stored. It is wasteful to get rid of the excess however, as amino acids contain useful energy. The nitrogen atoms are removed from the amino acide molecules in the liver. The amino acide molecule is kept, the nitrogen is excreted as Urea.
This process is deamination.
NH3 is removed from the amino acid, leaving C(R)OCOOH and NH3 (keto acid and ammonia)
The keto acid can be converted into a carbohydrate and used in respiration, or could be converted into fat and stored.
Ammonia is highly soluble and toxic. For this reason, it is immediatly converted to the less soluble and less toxic compound Urea
2NH3 + C02 ---> C(NH2)2O + H2O
An adult produces 25-30g a day
Humans also produce other nitrogenous waste compunds, such as creatinine and uric acid.
Metabolic reactions occuring within the body produce unwanted substances.
Some are toxic
Excretion if the removal of these waste products.
The two products made in greater amounts than most in the human body are CO2 and Urea.
* CO2 is produced continuously by every cell in the body by aerobic respiration The waste CO2 is transported via blood to the alveoli where it is excreted into the air that we breath out.
*Urea is produced un only one organ in the body: The Liver
Its is produced from excess amino acides and is transported from the liver to the kidnesy, in solution in blood plasma.
The kidneys remove urea from the blood and excrete it dissolved in water as urine.
[[Deamination]]
More Protien is eaten than is needed, the excess cannot be stored. It is wasteful to get rid of the excess however, as amino acids contain useful energy. The nitrogen atoms are removed from the amino acide molecules in the liver. The amino acide molecule is kept, the nitrogen is excreted as Urea.
This process is deamination.
NH3 is removed from the amino acid, leaving C(R)OCOOH and NH3 (keto acid and ammonia)
The keto acid can be converted into a carbohydrate and used in respiration, or could be converted into fat and stored.
Ammonia is highly soluble and toxic. For this reason, it is immediatly converted to the less soluble and less toxic compound Urea
2NH3 + C02 ---> C(NH2)2O + H2O
An adult produces 25-30g a day
Humans also produce other nitrogenous waste compunds, such as creatinine and uric acid.
Biology [[Control, Coordination and Homeostasis]] Homeostasis
[[Homeostasis]]
Maintaining a stable, internal environment. Internal environment is the conditions inside the body in which cells function. E.g. Tissue Fluid
The fuctioning of a cell can be determined by many things, including:
Temperature
* Low temps slow metaolic reactions, while high temps cause the denaturing of protiens and enzymes
Amount of Water
* lack of water in the tissues causes water to be drawn out of the cell by osmosis causing metabolic reactions to slow and/or stop.
* Too much water entering the cell can ccause it to swell and burst
Amount of Glucose
* lack of glucose causes respiration to slow and/or stop
* too much glucose may draw water in by osmosis
Homeostatic mechanisms wirk by controlling the composition of blood, which controls the composition of tissue fluid.
Most control mechanisms use a negative feedback control loop.
[[Negative Feedback Loops]]
The receptor picks up information about the parameter being regulated. This is known as the input. The input sets of a series of events culminating in some action by the effector. This is called the output. Continuous monitoring of the parameter by the receptor produces continuos adjustments of the output. This keeps the parameter oscilliating around an ideal level.
In negative feedback loops, a rise in parameter result in something happening which makes the parameter fall.
[[Positive Feedback Loops]]
There are a few instances of the opposite thing occuring in living organisms. If a person breathes air with a high percentage of CO2, there is a high percentage of CO2 in the blood. This is sensed by the carbon dioxide receptors, which causes breathing rate to increase. The person breaths faster, taking in even more CO2, which stimulates the receptors even more and makes the person breath faster. This is an example of positive feedback.
Positive feedback plays no role in keeoping things constant.
Maintaining a stable, internal environment. Internal environment is the conditions inside the body in which cells function. E.g. Tissue Fluid
The fuctioning of a cell can be determined by many things, including:
Temperature
* Low temps slow metaolic reactions, while high temps cause the denaturing of protiens and enzymes
Amount of Water
* lack of water in the tissues causes water to be drawn out of the cell by osmosis causing metabolic reactions to slow and/or stop.
* Too much water entering the cell can ccause it to swell and burst
Amount of Glucose
* lack of glucose causes respiration to slow and/or stop
* too much glucose may draw water in by osmosis
Homeostatic mechanisms wirk by controlling the composition of blood, which controls the composition of tissue fluid.
Most control mechanisms use a negative feedback control loop.
[[Negative Feedback Loops]]
The receptor picks up information about the parameter being regulated. This is known as the input. The input sets of a series of events culminating in some action by the effector. This is called the output. Continuous monitoring of the parameter by the receptor produces continuos adjustments of the output. This keeps the parameter oscilliating around an ideal level.
In negative feedback loops, a rise in parameter result in something happening which makes the parameter fall.
[[Positive Feedback Loops]]
There are a few instances of the opposite thing occuring in living organisms. If a person breathes air with a high percentage of CO2, there is a high percentage of CO2 in the blood. This is sensed by the carbon dioxide receptors, which causes breathing rate to increase. The person breaths faster, taking in even more CO2, which stimulates the receptors even more and makes the person breath faster. This is an example of positive feedback.
Positive feedback plays no role in keeoping things constant.
Subscribe to:
Comments (Atom)















