Empirical Formula: Simplest ratio of atoms of each element in a compound
Molecular Formula: Actual number of atoms of each element present in a compound.
To find emirical formula: Compoun X is found to contain 40.0% carbon, 6.7% Hudrogen and 53.3% Oxygen by mass.
% Mass 40.0 6.7 53.3
/Ar 40.0/12 = 3.33 6.7/1 = 6.7 53.3/16 = 3.33
/smallest 3.33/3.33 = 1 6.7/3.33 = 2 3.33/3.33 = 1
Simplest 1 2 1
ratio
Empircal Formula = CH2O
Finding the Molecular Formula - you need to know the mass of the compound.
Work out mass of empirical.
Equation = Molecular Formula = mass of molecular/mass of empirical x empircal formula.
Example = mass of empirical = 12 + 2 +16 = 30
mass of molecular = 60
60/30 = 2 x CH20 = C2H4O2
Isomers: Compounds with the same molecular formula, but in which the elements are arranged differently
Structural Isomers: Compounds with the same molecular formular but with different structures and represented by different structural formula
Structural Formula: shows the atoms present and all of the bonds between the atoms
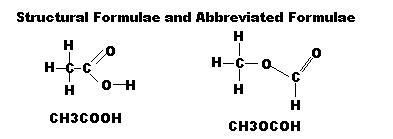
The abbreviated Formulae are used in equations, but Molecular Formulae should not be used. This avoids confusion.
Functional Group: atom or group of atoms which, when present in different molecules, causes them to have simlar chemical properties
Homologous Series: A family of molecules which all contain the same functional group and an increasing number of carbons
CnH2n+2 = alkanes
CnH2n = alkenes and cyclic alkanes
CnH2n+1OH = alcohols
All members of the same homologous series have similar chemical properties.
Their physical properties gradually change as the length of the carbon chain increases. For example, boiling point of alkanes increases as number of carbons increase.

No comments:
Post a Comment